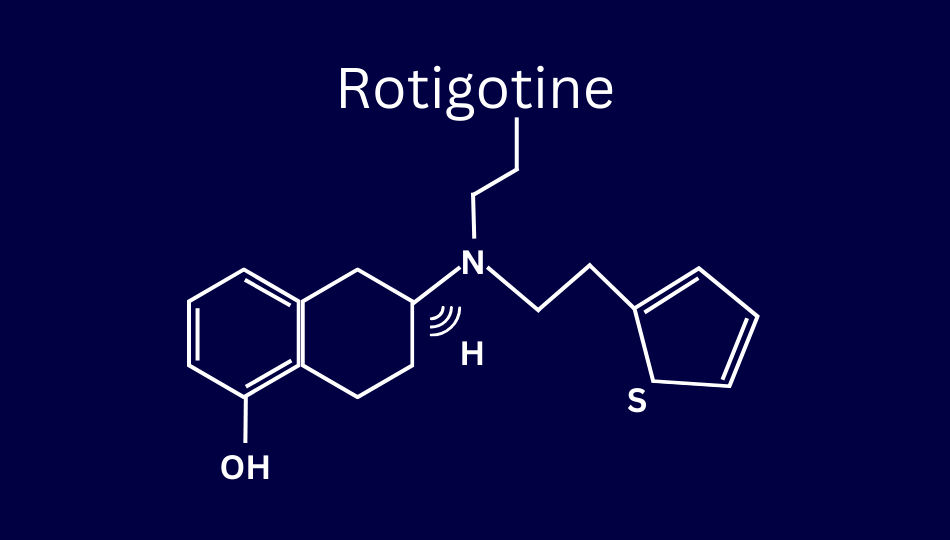
Rotigotine is a dopamine agonist delivered through the skin via an adhesive patch. This transdermal system delivers a steady supply of the medication for 24 hours. Rotigotine’s brand name is Neupro®.
The U.S. Food and Drug Administration (FDA) first approved rotigotine in 2007 to treat Parkinson’s symptoms. The drug is approved for use as a monotherapy in early Parkinson’s and concurrently with other treatments for advanced Parkinson’s. In 2008, the FDA approved the drug for treatment of Restless Legs Syndrome (RLS).
How Rotigotine Helps with Parkinson’s Symptoms
Like other dopamine agonists, rotigotine is designed to connect to the same receptors as dopamine. When you take this class of drug, the goal is to provide a synthetic replacement for the natural dopamine lost due to Parkinson’s.
One way to think about how this works is to think of dopamine and other neurotransmitters as keys. Just as each key has a corresponding lock, each neurotransmitter has a corresponding receptor. Dopamine agonists are chemical “keys” designed to fit the same “lock” as dopamine.
Because it is approved to treat both early and advanced Parkinson’s, rotigotine is available in multiple doses. The higher doses do not require more frequent application of a patch. Instead, the higher doses are delivered via a larger patch.
Reasons to Consider Rotigotine
While there are risks to rotigotine, there are many reasons to consider the drug to manage your Parkinson’s symptoms.
One Daily Dose
Ease of use is a considerable benefit of rotigotine: A single patch supplies 24 hours of medication. Moreover, because rotigotine is not taken orally, digestive issues do not influence the treatment’s efficacy. For example, you do not need to time your doses around meals, as is often necessary with oral medications like levodopa.
No Swallowing Required
Because rotigotine is not an oral medication, it provides a treatment option for people who have difficulty swallowing.
Improvements to Early Morning Motor Function
Many people with Parkinson’s spend part of their morning waiting for medication to take effect. The RECOVER study found rotigotine use corresponded with improvements in early morning motor function over a four-week period.
Help with Freezing of Gait and Reducing Fall Risk
In 2016, Ikeda et al. found rotigotine helped treat freezing of gait and reduced fall risk. The authors observed that the steady, 24-hour supply of medication may be a factor in this effect.
Possible Non-motor Symptom Benefits
In 2018, Valldeoriola et al. wrote that rotigotine reduced non-motor symptoms by 14.6%. Improvements were observed in rates of urinary, sleep, and mood/cognition-related symptoms.
In 2020, Castrioto et al. wrote that rotigotine was associated with improvements in levels of anxiety.
Evaluating the RECOVER study, Kassubek et al. observed that rotigotine was associated with decreased pain.
Efficacy and Risk of Rotigotine: Data from Clinical Trials
In 2007, Jankovic et al. wrote, “Rotigotine consistently demonstrated statistically significant and clinically relevant efficacy over placebo in patients with early PD, as assessed by significantly greater improvement with time in the UPDRS subtotal scores (parts II and III) and UPDRS parts II and III individual scores.” The authors also wrote that once-daily use of transdermal system provided stable levels of medication in participants’ blood samples over a six-month period.
In 2013, Zhou et al. published a meta-analysis of research about rotigotine. The report’s core findings include that rotigotine significantly reduced the symptoms of Parkinson’s compared to placebo. The report says application-site skin irritation, nausea, and sleepiness were the most common side effects.
Other findings from the 2013 meta-analysis include:
- Participants with advanced Parkinson’s experienced significant decrease in OFF-time without increasing troublesome dyskinesia.
- The degree of improvement seen in UPDRS rating scores was lower for rotigotine than for other dopamine agonists in previous trials, but this may be explained by shorter duration of rotigotine trials.
In 2021, Ruan et al. compared the efficacy of multiple dopamine agonists in people with Parkinson’s experiencing motor fluctuations. Their core findings include:
- Rotigotine was the second-most effective of six dopamine agonists in terms of UPDRS part III scores, although there was not a significant difference between rotigotine, ropinirole, or pramipexole.
- Rotigotine was associated with greater increase in quality of life than other dopamine agonists, as measured by UPDRS part II rating scores.
- Rotigotine was one of three dopamine agonists with significantly higher rates of gastrointestinal side effects.
Rates of incidence of Nausea and Vomiting
Regarding gastrointestinial side effects, the FDA’s prescribing information notes that in clinical trials rotigotine was associated with increased rates of vomiting and nausea compared to placebo.
Rates of Nausea According to FDA Prescribing Information
In a trial for people with early Parkinson’s:
- 13% of those receiving placebo experienced nausea.
- 34% of those receiving a 2 mg dose of rotigotine experienced nausea.
- The incidence rate was higher for those who received a 4 mg (38%) or a 6 mg (48%) dose of rotigotine.
In a trial for people with advanced Parkinson’s:
- 19% of those receiving placebo experienced nausea.
- 28% of people receiving an 8 mg dose of rotigotine experienced nausea.
Rates of Vomiting According to FDA Prescribing Information
In a trial for people with early Parkinson’s:
- 3% of people receiving placebo experienced vomiting.
- 10% of people receiving a 2 mg dose of rotigotine experienced vomiting.
- The incidence rate was higher for those who received a 4 mg (16%) and a 6 mg (20%) dose of rotigotine.
In a trial for people with advanced Parkinson’s:
- 6% of people receiving placebo experienced vomiting.
- 10% of people receiving an 8 mg dose of rotigotine experienced vomiting.
Importantly, in discussing an early trial for rotigotine as a monotherapy, authors from the Parkinson’s Study Group write, “Nausea and vomiting occurred primarily during the dosage titration phase, with a trend toward symptom onset at the time of initiation of active drug.” This suggests these side effects may be temporary. Moreover, when analyzing data about adverse events associated with rotigotine, in 2013 LeWitt et al. found most occurrences of nausea were considered mild.
Other Risks of Rotigotine
Like other dopamine agonists, rotigotine is more likely than some other Parkinson’s medications to cause or worsen impulse control disorders (ICDs). In 2016, Antonini et al. wrote that 9% of participants in long-term use trials of rotigotine experienced an ICD.
In their 2013 article, LeWitt et al. examined research about long-term rotigotine use by people living with advanced Parkinson’s. The authors evaluated data about adverse events (AEs) from two open-label studies. The table below highlights some of their findings:
| Adverse Event (AE) | Participants in Trial One Experiencing AE | Participants in Trial Two Experiencing AE | Average % of AEs Considered “Mild” |
| Application Site Reaction | 26% | 33% | 71% |
| Fall | 16% | 40% | 50% |
| Hallucination | 9% | 23% | 50% |
| Sleepiness | 34% | 58% | 72% |
| Decreased Weight | 11% | 9% | 62% |
The authors mention other side effects as well, and summarize the AE profile of rotigotine by saying, “With the exception of application site reactions, a known side-effect of the rotigotine patch, the adverse event profiles were similar to those observed in previous long-term studies of other dopaminergic agonists.”
The FDA’s prescribing information also references other risks, including:
- Rotigotine may cause allergic reactions, including anaphylactic or asthmatic episodes in people with allergy to sulfites.
- Rotigotine may contribute to blood pressure variation.
- Rapid dose reduction may cause elevated temperature, significant rigidity, and other autonomic symptoms.
Rotigotine: A Unique and Valuable Treatment Option
While it will not be the best option for everyone, rotigotine is worth discussing with your care team to see if it might be right for you. The medication is approved for use as a monotherapy and as an add-on therapy. Therefore, rotigotine can be a part of your long-term treatment plan and help you remain active and live well for many years.
Despite the possible side effects, rotigotine’s ease of use and unique, transdermal delivery system make it a valuable treatment option. Because rotigotine supplies a steady supply of medication, it can help treat multiple complicated Parkinson’s symptoms—including gait disturbances and sleep related issues—with a single dose, all day long.
ADDITIONAL RESOURCES
2007 Rotigotine Study Report in Jama Neurology
Press Release About Availability in Europe
Canadian Drug Expert Committee Report
Davis Phinney Foundation Medication Guide
HAVE YOUR GIFT MATCHED THROUGH THE END OF THE YEAR!
 Our End of Year Giving Campaign is underway, and we need your support to continue providing our resources and programs to people who need them. We have more lives to impact and your support will help us do that. Will you join us by donating now?
Our End of Year Giving Campaign is underway, and we need your support to continue providing our resources and programs to people who need them. We have more lives to impact and your support will help us do that. Will you join us by donating now?