Deep brain stimulation (DBS) is a highly effective and reasonably safe treatment for Parkinson’s; however, it’s also frequently misunderstood. The success stories about how people are sometimes living symptom and medication-free as a result of getting DBS understandably lead many to want to get the treatment as soon as possible. Others think it sounds scary and shy away from it. During this webinar, Dr. Joohi Jimenez-Shahed broke down the what, why, and how of DBS and its latest advancements.
Watch the recording below.
To download the audio, click here.
You can read the transcript below. To download the transcript, click here.
Leigh Cocanougher (Education and Research Program Manager, Davis Phinney Foundation):
Hello and welcome everyone to the October live well today webinar from the Davis Phinney Foundation. Today, we’re going to be talking about the latest advancements in deep brain stimulation. I’m Leigh Cocanougher, the education program director at the Foundation. And before I pass it over to our moderator today, Dr. Soania Mathur, I want to thank today’s gold sponsors, Abbott and Medtronic, and also our peak partners, Adamas, Amneal, Kwoya Kirin, and Sunovion. Thank you for supporting this educational content and making it possible for us to provide it free to the Parkinson’s community. And now, Soania, I will hand it over to you. Thank you.
Soania Mathur, MD (Board of Directors Member, Davis Phinney Foundation):
Thank you so much Leigh, good afternoon, everyone. Thank you for joining us for today’s webinar, deep brain stimulation, and Parkinson’s the latest advancements you need to know. As we mentioned, I’m Dr. Soania Mathur, I’m a family physician. I’m also a Parkinson’s patient for the last 22 years, and a very proud member of the board of directors for the Davis Phinney Foundation. And I have the very distinct pleasure of being your moderator today. As you know, unfortunately there’s currently no cure for our disease. Otherwise we wouldn’t be here, but there are a variety of treatment options that our medical team can offer us directed at managing our symptoms, minimizing side effects from our medications, and really optimizing our quality of life. Because until there’s a cure, it’s really all about quality of life and the treatments that will give each of us the best life experience really do differ from individual to individual.
So in conjunction with our medications, there are surgical options such as deep brain stimulation that can help manage our Parkinson’s symptoms. But of course, these procedures are not for everyone. So we will discuss a bit about what this procedure is all about, the history of DBS and how the technology is advancing, which it is. So to help us further explore this interesting topic is Dr. Joohi Shahed. She is among many things, the medical director of movement disorders, neuromodulation and brain circuit therapeutics at Icahn School of Medicine at Mount Sinai. After completing her undergraduate degree at Washington University, Dr. Shahed received her medical degree from Baylor college of medicine and neurology residency training at Duke University. She then completed a fellowship in movement disorders at the Parkinson’s Disease Center and Movement disorder Center at Baylor. Her research interests lie in investigating the interoperative neurophysiology of patients undergoing DBS.
So that’s basically looking at what patients’ brains are doing, I guess, during the procedure and the application of wearables and digital health technologies to the care of patients with Parkinson’s. She’s also the chair of our scientific advisory board here at the Foundation, Dr. Shahed, thank you so much for joining us today.
Joohi Jimenez-Shahed, MD (Medical Director of Movement Disorders Neuromodulation & Brain Circuit Therapeutics at the Icahn School of Medicine at Mount Sinai):
Thank you, Soania. It’s great to be here.
Soania Mathur:
So most people at either having listened to previous webinars, or just in general, have heard about DBS and probably know involves some sort of brain surgery, but may not be really familiar with the details. So could you please maybe start off by giving our listeners a better idea of what DBS is and how we think it works?
Joohi Jimenez-Shahed:
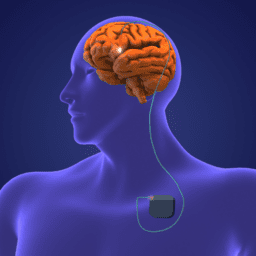
Yeah. that’s a great place to start. So deep brain stimulation is a surgical therapy, as you mentioned, it involves the implantation of hardware. So there is a device associated with deep brain stimulation. You can think of it kind of like a pacemaker. So a pacemaker has a battery. The battery has wires. The wires are connected into your heart to make sure that the heart rhythm continues to function normally. And then similarly with the deep brain stimulator, you have a battery, you have wires, but this time the wires are connected into those deep brain structures that are responsible for controlling our movements. And what we know about the movement circuitry in the brain in patients with Parkinson’s disease is that the brain signals, those circuits just start misfiring and they’re not working properly. And when we can go in there with a little bit of electricity with the deep brain stimulation device, then we can kind of reset those circuits a little bit and get them to behave a little bit more normally and thereby improve the symptoms of Parkinson’s disease. And so that’s really kind of a, I think a sort of a general overview of how that therapy can be used to improve symptoms that patients are familiar with such as stiffness or slowness or tremors or walking and balance.
Soania Mathur:
Right. That’s a great sort of overview of it, but what, how does a patient know they’re ready for DBS? Like what kinds of patients do you look for who tends to have the best outcome and that sort of thing?
Joohi Jimenez-Shahed:
Right. So I think you know, DBS is a great therapy, but it’s a great therapy for the right patient at the right time. And I think that’s sometimes the hardest part of this to really sort of convey or to help people kind of wrap their heads around. So we know that when we’re treating Parkinson’s disease with medications over time, you know, initially patients obviously can have a great response. They can function pretty well, but then over time they start developing the feeling that the medications are wearing off or they might start to experience dyskinesias, and often they’re experiencing both. So you take a dose of medicine, it kicks in, you start having dyskinesia, it starts wearing off. And you’re kind of doing this up and down fluctuating all day long. The other situation where we have difficulty with medication management is when patients have a lot of tremor, sometimes patients with really tremor dominant symptoms in their Parkinson’s disease, it’s a little bit more challenging to manage that tremor completely or sufficiently for, you know, their quality of life, as you mentioned.
And these are really the two most common situations where we start thinking about doing DBS. So if somebody’s medication effects are wearing off and they’re having dyskinesias, or if they’re having this tremor that really can’t get sufficiently controlled with medications, that’s usually the time that we’re thinking about doing the DBS. And I think when we sort of stick to our sort of rigorous evaluation processes for DBS, then we can really kind of ensure that patients are going to have the best outcome as possible.
Soania Mathur:
And through those evaluation processes, what are you looking for in terms of sort of that ideal candidate?
Joohi Jimenez-Shahed:
Yeah, I think you know, Parkinson’s is predominantly a condition where people have difficulty with their movement, but we know that there are a number of non-motor symptoms that can occur with Parkinson’s disease as well. And so these are things like maybe depression or other mood changes, perhaps even some cognitive difficulties. And while there’s a whole host of other non-motor symptoms that can happen in Parkinson’s, these are usually the ones that can have significant impact on how somebody does with deep brain stimulation. And so, although none of like, you know, cognitive impairment or anxiety or depression, aren’t necessarily reasons not to do deep brain stimulation, there are definitely things that we need to be aware of prior to going into the whole process of DBS for a couple of reasons. I think we learned early on with DBS that if we operate on patients who already have substantial cognitive impairment, there’s a risk of that further declining after brain surgery.
So we want to be very cautious in situations like that because we don’t want our patients to get worse. In the case of depression and anxiety, there have been situations where when those symptoms are either unrecognized or not very well controlled, that they have also kind of spiraled out of control for patients. And so what we do when we evaluate people is not only give them a very careful assessment of the motor symptoms, like what happens on medication or off medicine, but then we also do a neuropsychological evaluation and in some centers such as ours at Mount Sinai, we also do a more elaborate psychiatric and psychological evaluations prior to surgery. And this is really to try to understand, is there depression, that’s maybe influencing how this person is functioning or feeling? Is there anxiety that might make it hard for individuals to go through the whole process of deep brain stimulation? And so, we try to get a handle on that so we can do our best to manage it alongside the whole process of surgery. Again, as I said, it’s not always a reason not to do surgery, but it’s definitely something that we want to assess, make sure we’ve got good control or as much control as we can prior to going through the entire procedure.
Soania Mathur:
So you’ve mentioned the cognitive issues and the mood issues that might preclude someone from benefiting or going through DBS. Are there other contraindications that you would say this person should not have DBS?
Joohi Jimenez-Shahed:
So I think you know, the main issues should be that the individual aside maybe from tremor, that’s difficult to control with medications should have a Parkinson’s that is responsive to the levodopa. In other words, you should be able to take a dose of medication. It should be able to help your symptoms, but then you’re dealing with these complications like the dyskinesias and the fluctuations. I think aside from that, other sort of contra-indications may not really be specific to Parkinson’s, things like somebody who maybe has a lot of other medical conditions that might make it hard for them to go through surgery. Some people, especially in this kind of age range where Parkinson’s is also a problem, maybe on blood thinners, for example. And so we have to make sure is it possible for individuals to be off their blood thinners while they undergo brain surgery? So some of those types of issues do come up from time to time. And that’s all part of our sort of pre-surgical evaluation as well.
Soania Mathur:
Is there an age limit Dr. Shahed?
Joohi Jimenez-Shahed:
Yeah, that’s also a good question. I think you know, early on again, we had broadly applied deep brain stimulation to anybody who’s sort of met some of these criteria. And I think there maybe have been some suggestions in the past that people who are older, or maybe even after the age of 70, I think that number has been tossed around a fair amount in the literature you know, should not get DBS or might be, you know, have at risk of having lesser outcomes from surgery. But I think really, you know, we’ve operated on young patients, and we’ve operated on older patients. And I think if we apply again, our criteria in terms of making sure this is truly, you know, a patient with levodopa responsive Parkinson’s or somebody with fluctuations, dyskinesias, or refractory tremor without the cognitive issues or significant psychiatric issues, the age doesn’t necessarily have to be a major consideration in terms of whether or not somebody is a good candidate. I think we really need to think broadly about kind of the big picture of that individual.
Soania Mathur:
Right. And I think you mentioned this, but just to maybe reiterate it, the DBS targets sort of more the motor symptoms of Parkinson’s disease, specifically the tremor, but we know that non-motor symptoms of this disease can be really detrimental to patients’ quality of life. Are there any non-motor symptoms that can improve with DBS?
Joohi Jimenez-Shahed:
Yeah, I think this is really an interesting area because you know, when we start evaluating patients prior to surgery, we assess their symptoms on medication, and we assess their symptoms off medication. But if you look really closely beyond things like the routine motor symptoms, like the tremor or the rigidity, or the bradykinesia, or the gait, you can also see that there are patients who are maybe much more anxious when they’re off their medications, or they are, you know, less cognitively maybe just have a little bit more difficulty processing information when they’re off medications or maybe their mood is clearly different. And there’s a number of different symptoms like that. Even things like bladder control can be worse off medications. And so what we generally tend to see when we do deep brain stimulation is that we minimize those OFF times.
So when all of these symptoms tend to be worse in the off state, they can, as a consequence, improve to a certain degree just with the DBS themselves, because we are better able to keep patients ON more of the time after surgery. So there definitely can be some improvements. I think some of the biggest areas might be in those really obvious, you know, situations where patients are, like I said, anxious, or just maybe their mood changes a little bit or the sleep issues. And I think bladder issues, these are probably some of the major areas where patients might have notable differences.
Soania Mathur:
Well, that’s actually huge and it really makes DBS an interesting addition to treatment regimen, for sure. So from what I understand it was in, I think 2002 that DBS for the treatment of Parkinson’s was granted approval by the US food and drug administration, but neurostimulation was sort of being treated, used to treat a range of other conditions starting, I think in the early 1970s or something I thought I read replacing the surgeries that they used to ablate brain tissue, which is lesioning the brain tissue to treat certain symptoms. So maybe, it’s a very interesting read if anyone has time, but can you walk us through some of the history, like how DBS came about?
Joohi Jimenez-Shahed:
Yeah, I mean, I think the initial sort of idea that these parts of the brain are even involved in controlling Parkinson’s symptoms was actually just a very accidental finding. So there were patients who were getting surgeries for other things, and they may have had sort of an interoperative inadvertent kind of stroke that actually happened in precisely the areas that we now use to stimulate the brain. And what was found in those patients is that when those strokes happened in those areas, their Parkinson’s symptoms actually improved. And so that was really kind of some of the foundational basis of understanding the circuitry that’s involved in Parkinson’s disease and how we might be able to affect that circuitry by surgery. And so you’re right, the kind of initial attempts were actually at lesioning. So lesioning means going in, and at that time it was burning a little hole.
So you did it you know, with the same kind of wire, but you kind of gave those high intensity electricity to kind of create a little area of damage in a very precise location so that it would help the Parkinson’s symptoms. And it did for a number of patients. Unfortunately some of the challenges with those lesion therapies were that the effects were not long lasting. And so patients may have had initial benefit, but then over time, the brain sort of learned how to you know, kind of accommodate or adjust or somehow overcome the effects of those lesions and patients would continue to still have problems afterwards. They were also limited by the fact that you really couldn’t treat both sides of the brain in that way. So if you had tremor on one side, great, we could maybe address that, but the symptoms on the other side of the body might still be problematic.
And I think, you know, along the lines, people started thinking about this idea of stimulation and applying the electricity to the circuitry that way, as opposed to damaging the circuitry with the recognition that something like electricity is modifiable. And so you can adjust it over time. So if there does become a situation where symptoms worsen, then you could maybe ramp it up a little bit without having to do another surgery. And I think as kind of experience grew with it initially it was being offered just on one side of the brain, but then eventually both sides were treated, and it was found that people treated with deep brain stimulation on both sides of the brain did far better than patients who were treated with lesions on both sides of the brain in terms of the side effects and the outcomes and things like that. And so that’s kind of how it grew. So part of it was just through, you know, just kind of accidental findings and part of it was through experimentation and trying to see how did patients really react and then really getting hands on experience treating patients. And I think over time, we’ve just seen this incredible evolution to the point that we’re at today which is just really sort of amazing technology to deliver this type of therapy.
Soania Mathur:
Yeah, it’s really interesting how many things in medicine have sort of been found by happenstance and just, you know, acute observation of what goes on naturally sometimes. To talk about DBS specifically, I know there’s, I believe three main companies that make devices each varying a little bit from each other, what types of DBS systems are available for patients today?
Joohi Jimenez-Shahed:
So all three manufacturers of devices, they essentially work in the same way in the sense that they have a battery. The battery is connected to the wires, the wires and the deep brain structure. And you are able, the clinician is able to manipulate the way that that electricity is delivered in order to get the best results for their patient. So in that sense, they all have the same basic components. All of them also come with a patient programmer and things like that. So the hardware from that very basic standpoint is quite similar. But the way that I like to explain it is that the bells and whistles in that hardware are a little bit different from from manufacturer to manufacturer. And so you know, the batteries, I think are one of the places that really we see the biggest difference.
So some batteries are capable of delivering stimulation in a way that you can control in a precise way in terms of creating this field of electricity, others allow you to steer the current in particular directions. And yet a third device allows us to actually sense the brain signals and use that information in order to program the device. So there are, you know, I think from the standpoint of differences, mainly amounts to how the clinician can use the device most effectively to control the symptoms that a patient is having.
Soania Mathur:
So who gets to decide which device, is that, does the patient have input or is it usually up to the surgery, or the surgeon usually?
Joohi Jimenez-Shahed:
Yeah, I mean, I think that’s a, it’s probably different from place to place to be very honest with you. And I think I always encourage patients to be familiar with what’s out there. So they kind of understand a little bit about the device and how it can work and, you know, if they have preferences, they should certainly be able to voice those. I think sometimes there are matters perhaps a little bit beyond sort of individual control that do determine what devices are available at individual centers. So for example you know, a hospital may have a contract with one of the manufacturers and so that’s the device that they have available. And so you know, and that may have been driven by a variety of different factors, but sometimes that is kind of the reality. And so if this is you know, if there’s a particular device that a patient is interested in, I think they can certainly have that conversation with the neurologist and the neurosurgeon that they’re working with to find out what’s available and how that device is being used at that particular center, or if they feel very strongly about it could certainly find, go out and look for a place that does use that device.
So I think it’s important for people to feel comfortable with what treatment they’re getting. I do want to emphasize though that all of these devices are going to work as long as you have a good surgeon and a good programmer. They’re not necessarily that different in terms of you know, getting the results that we generally anticipate patients to be able to get.
Soania Mathur:
So are there any specific criteria other than patient preference, surgeon being comfortable with one device or center availability that are used, are there specific criteria that are used in making those decisions between advices, between devices? Sorry.
Joohi Jimenez-Shahed:
Yeah, I mean, I think certainly not any, you know, it’s not like patients with a certain type of Parkinson’s or with certain symptoms of Parkinson’s would benefit from one device or the other, like I said, anybody can get really good benefits from any of the devices. And I think that’s a, you know, that’s a great statement to be able to make actually. So we do have variety in our choices, and it may boil down to different things like you know, maybe somebody really wants to have a rechargeable battery. And so a couple of the device manufacturers have a rechargeable battery that’s available and maybe somebody, you know, prefers a certain platform they’re all on different, the programmers that the patients get back are actually on slightly different technology platforms as well. And so there might be some of these things that are individually important, you know, when patients do their research that they can find out about, and maybe they kind of like that, or maybe their neurologist, you know, really finds it helpful to be able to use those particular features when they’re managing their patients.
I think, you know, some of the most important decisions that a person can make as they’re seeking DBS therapy. Yes. There’s the device question. I think the biggest question is, is DBS, right for me? Am I ready to do it? Secondly, do I feel confident in the person who is taking me through this journey, the neurologist, as well as, you know, the neurosurgeon and you know, do they really have that level of disability that this is really kind of the right time to be doing that procedure?
Soania Mathur:
You just actually touched on something I always sort of hope to give our listeners some practical advice in these webinars. And so we’ve learned a little bit about the information about the procedure procedure itself, although we’ll talk more about that and the types of systems that are available, but how does one go about finding a neurosurgeon to actually perform the surgery? Cause it’s not a simple task, it’s fairly invasive and significant as a procedure. So what should patients be asking their potential surgeon?
Joohi Jimenez-Shahed:
Yeah, I think hopefully most patients with Parkinson’s are being managed by a neurologist and usually that neurologist will have a relationship with the surgeon who performs that procedure or has familiarity with one. I think some of the questions to ask are whether, you know, that like how many cases have they worked on together or specifically how many patients are in that neurologist’s practice. But then from the surgeon’s perspective, I think you know, there are surgeons who do a lot of DBS surgeries and some surgeons who do fewer surgeries. And so you want to get a sense of their familiarity with that procedure, the number of procedures they’re doing per year. You can also ask them about their complication rates and about any side effects or other experiences that they may have had with with the DBS patients, all surgeons sort of keep track of these things and they’re able to provide that information regularly.
And I think through that kind of conversation, you can get a sense of you know, how experienced that surgeon is and, and what level of confidence you have in that individual. And I think it’s good to have those conversations, but I also think it’s an it’s important to make sure that your team is working well together so that the neurologist can communicate with the surgeon and vice versa so that, you know, it’s a long-term procedure. It’s not just go get the surgery and get it done and come back and you know, everything. And it’s like done at that. There’s device maintenance, there’s maybe battery replacements. So you’re going to have relationships with both of these types of doctors over the long-term. So you need to be comfortable with them.
Soania Mathur:
Yeah, no, I agree. I think that those questions and conversations are really important, and people shouldn’t feel shy or awkward in asking those questions. I mean, like I said, this is a fairly significant procedure that you’re contemplating, and you have to be, as you said, very comfortable with your whole team. And you mentioned batteries just to go back to that. I know there’s sort of rechargeable ones and non-rechargeable or fixed ones. What are some of the pros and cons of each of those?
Joohi Jimenez-Shahed:
Yeah, I think yeah, there’s different conversations that can be had about a battery. So some batteries are shaped a little bit differently than others. And so that may have certain impacts. The recharge, well, so the conventional battery life depends a lot on you know, the programming that’s required to control the symptoms. So if you think about it, the more juice you’re needing in order to control the symptoms, the faster it’s going to drain that battery. So on average, we say about three to five years in terms of a conventional battery. So if you’re going in and sort of thinking about doing it that way, that’s about the timeframe that you would expect to have to go through a battery replacement. The rechargeable technology has actually advanced significantly in the last few years, and now the rechargeables that are on the market are actually good to go for 25 years which is really kind of amazing.
The rechargeable batteries are smaller. So I think from a sort of patient comfort level, appearance, those kinds of things, there may be some advantages to the rechargeables, but the trade-off is the maintenance of the charging. And you know, most people need to plan on charging at least once a week in order to keep their therapy going. Once you let the battery go down a little bit too much, then even before it goes completely out, there might be some changes in symptom control. So you have to really think about just, you know, being in that mindset of being able to regularly sit and charge that on a regular basis. That’s really kind of the main trade-off. I think as we grow in our world with different kinds of technologies, we’re seeing a lot of increasing familiarity and comfort with that. And so, I think a lot of people are doing quite well with the rechargeables, especially because the charging systems are so much more user-friendly these days than they used to be.
Soania Mathur:
And that can be done at home.
Joohi Jimenez-Shahed:
It can be done at home. Yeah. And you don’t have to sit plugged into a wall. Usually there’s a, there’s kind of a charging apparatus that you can kind of hold on yourself in a, not too terribly burdensome way. And so you can actually be up and around and doing things if you so choose. You know, sometimes the amount of time that you have to spend charging depends on how well you can make contact with your battery that’s in you using the charging device. You just want to be sure about that, but you don’t have to be restricted or you know, tied to something during the process of charging.
Soania Mathur:
That’s good to know. I have trouble plugging in my car. So I would worry about that. If you went for a non-rechargeable one you mentioned it has to be changed at some point, how long, what’s that like, how long does it take to reach that point? And what is involved with that?
Joohi Jimenez-Shahed:
Yeah, so again, depending on the setting, so the higher the settings, the shorter the battery life, but generally speaking for what we know tends to be required in patients with Parkinson’s, we’re thinking about three to five years before you need the battery exchanged. The battery exchange itself from a surgery perspective is not too terrible. It’s a day surgery. So, and it’s usually not even done under general anesthesia. Usually it gets some conscious sedation, something to make you comfortable and relaxed, and that’s a local anesthesia. So the surgeons will go into the same place that you have, the original incision. They will take out the old battery, disconnect it, clean things up, put in a new battery, stick it back in, sew it back up and really that’s maybe about an hours worth of procedure. So not a kind of full-fledged and certainly not a brain surgery to get the battery replaced. So it’s definitely doable.
Soania Mathur:
That’s good to know. This question has come up in our chat, but also anecdotally I’ve heard that sometimes it can take a little bit of time to get things programmed properly in order to alleviate the symptoms and kind of adjust to that. Can you tell us a little bit about that procedure and what normally people should expect?
Joohi Jimenez-Shahed:
Yeah, I think that’s a really good question. An important one. So I’ve alluded to now a couple of times that there’s a whole process of DBS and half that process is making the decision to get it and then getting all your pre-op assessments, getting your surgery, but then the real work begins after that, which is getting, you know, the device to control the symptoms the way that you need it to. And it’s not as simple, just turn it on and set it and forget it kind of situation. Right. So the electricity that we’re applying in the brain works together with the medication. But the idea is that as we start improving Parkinson’s symptoms with the stimulation, then we can start reducing the medications. And you don’t want to do either of these things too quickly. The brain may not respond very well to stimulation that’s titrated too quickly, or to medications that are reduced too quickly.
So you kind of have to do a little bit of programming, wait to see what that does, a little bit of medication adjustment, and then go back to the stimulator and adjust that. And then maybe some medication adjustments and vice versa, kind of ongoing for a little bit of time. And I think depending how your neurologists handle it, because I think different neurologists might do this differently. I can tell you in my practice, I do this on a monthly basis. So I’ll, you know, once I have a surgery date for my patients, then I line them up for six months of programming. And different physicians might do that differently, but you should expect that it takes at least four, five, six sessions to really kind of iron out the settings. And I think if you sort of take that approach, usually by the end of those six months, we can find some stability and we can find kind of that new level of normal that patients can expect from deep brain stimulation.
Soania Mathur:
What about those in our community that might be more geographically remote and underserved? What are their options? If it requires lots of travel, is there anything else that they can do?
Joohi Jimenez-Shahed:
Right. So this is kind of an emerging and exciting area in DBS. And I think if COVID taught us anything, it was that we could actually deliver health care remotely. The technology had to catch up with that a little bit, and thankfully there is a device manufacturer that does have a remote programming capability. And so for individuals who have that device, if they’re connected to a center that is able to use that remote programming capability, they can actually sit in the comfort of their house while the physician is at the office and have a remote connection over the internet. It is done very securely with a lot of checks and balances to make sure that it’s also done very safely. And those individuals can be programmed that way. Now, what it does require is a good internet connection.
And so if you are in one of those sort of remote locations where there may not be as good or reliable internet, that might still be a bit of a challenge, but that is definitely a possibility. I think other things that are also available across devices is patient control parameters. So everybody gets a controller and depending on the situation of the individual patient or the symptoms that they’re experiencing the clinician may be able to program in some opportunities for the patient to adjust at home. And one of the things that we did as a center during the COVID crisis, when everybody was at home and not wanting to come into the doctor was liberalizing our amount of patient control that was available. And then through regular video visits, we would just say, okay, click it up, click it up, let’s see what happens, click it down, trying different options that way. And so that is also a possibility that that can be done.
Soania Mathur: That’s exciting.
Joohi Jimenez-Shahed: And really neat actually.
Soania Mathur:
Yeah, it is, very futuristic. What are some of the limitations of the technology then? I mean, some cannot be remotely adjusted, but in general, what are some of the limitations that we have with DBS at the moment?
Joohi Jimenez-Shahed:
So DBS, you know, probably the most important thing to recognize is that it’s not going to be a replacement for your medication. So it is going to help control those symptoms for the most part, helping reduce the OFF times and increasing the amount of time that somebody has ON. It also should reduce the dyskinesias, it should help control the tremor. Really anything that can get better with levodopa should get better with deep brain stimulation. And so we can get somebody who was kind of doing it up and down fluctuating all day to be a lot more steady during the day. And that has been, you know, one of the greatest sort of advantages to the stimulation. Having said that, most patients do still have to take medication. We can reduce medications, differing amounts, depending on the location of the stimulation.
But the other kind of limitation is that we know that Parkinson’s is progressive. So just because we put in a stimulator, it doesn’t mean that we’ve stopped Parkinson’s. Unfortunately things will continue to evolve. And to a certain extent we have the capability of adjusting the stimulator to recapture some of that control, but there are some symptoms that might continue to progress. And these are mainly things like speech issues can sometimes be very challenging, balance issues, certain walking issues, especially gait freezing, might become challenging to control over time just with the stimulator adjustment. So we might have to use different strategies to manage those things. And when we’re kind of approaching patients in the long-term management of their Parkinson’s with the stimulator, one of the first things we have to decide is, is the problem that somebody is coming to me with something that I can fix by adjusting the stimulator or not. And sometimes the answer is yes, and sometimes the answer is no, and it’s really, you know, you have to figure that part out and try, you know, kind of the whole range of treatment strategies for some of these symptoms.
Soania Mathur:
I mean, you’re right. I mean, DBS, as you mentioned, is a symptomatic treatment, doesn’t treat Parkinson’s itself and that continues unfortunately to progress, but patients sort of do well. Is there a number of years that you say that, is there a kind of an average number of years that patients can expect to do well after the DBS?
Joohi Jimenez-Shahed:
So, you know, there have been now some long-term studies that have been published on patients who have had simulation for, you know, 15 years, for example. And some of the things that we talked about as far as the initial reasons for doing DBS, whether it was the fluctuations, the dyskinesias, the tremors, these are all things that can remain very well controlled over that timeframe. So those initial benefits can be maintained through the long-term and, you know, there’s always going to be individual variances. So it’s hard to be precise in terms of exact numbers of years. But it’s always, and it never ceases to amaze me. You know, sometimes we end up in a situation where we have to see what happens when you turn the stimulator off and even, you know, 10, 15 years down the line, you can see some very obvious differences in patients without their stimulation. So I think there are some long-term sustained benefits sort of combined with that though, is the eventual progression of symptoms. And so there might just be a new set of symptoms that people experience that as I said, we can’t really control as well. So there’s not really, you know, I don’t think there’s really like a time limit on the amount of benefit that patients can get from it.
Soania Mathur:
What about early in disease? One person in the chat asked about that, and I’ve also heard that sometimes there’s maybe an advantage of getting DBS earlier in the course of disease. Can you tell us a little bit about that, is that true?
Joohi Jimenez-Shahed:
Yeah, I think you know, again, when we first started out, you talked a little bit about this history it’s been around since the nineties and, you know, it was sort of the thing you did at the end of everything else, right? So you’ve tried your levodopa, you’ve tried this, you’ve been doing it for tens of, you know, however many years and then, you know, do surgery. It was kind of often viewed as sort of a last resort. And I think slowly we’ve realized that it doesn’t have to be the last resort. Our current indication, the most detailed explanation of that indication is actually an individual with Parkinson’s for at least four years who’s experiencing troublesome motor fluctuations and dyskinesias, so it’s very vague in that sense on purpose. So it gives us some flexibility. And so we’ve definitely seen over time that we’re doing it kind of earlier in the disease course, but I still think that, you know, the main issue should still be there in terms of the feeling that the medications are wearing off or the dyskinesias, or the tremor that’s not adequately controlled, I think less than four years is still uncertain territory.
And there have been some studies that have been done in patients who were maybe a year or two out from diagnosis of Parkinson’s disease, very, very early in their symptoms. And while those patients did benefit, it’s not yet clear whether there is an actual advantage to doing that and exposing patients to risk of surgery that early in their disease course. And so that hopefully is information that we will continue to try to find out over time, but we’re not quite yet there to be thinking about it right out of the gates, if you want to say it that way.
Soania Mathur:
So patients don’t have to sort of be at the end, or maxed out on their medications before they start discussing this possibility with their neurologists.
Joohi Jimenez-Shahed:
No, they really don’t. And I think you know, one of the things that even some of the earliest studies in deep brain stimulation showed us was that even if you continue to manage medications for patients, there might be some things that we can achieve with medication management. Maybe we can reduce some of the OFF time, maybe we can improve the dyskinesias and things like that. I think there’s sort of this balance that you have to achieve where you felt like you’ve tried a sufficient amount of those to accept the risks of brain surgery. I mean, it’s still brain surgery. And so the risk profile is very different from continuing to take medications, but even in those early studies, you know, the studies demonstrated that surgery is better than medication management. So if you have a patient who’s dealing with those symptoms and you continue to manipulate medications for an extended period of time, you really don’t get much more advantage as a group compared to going ahead and offering those patients surgery.
And I think there’s increasing recognition of that. And that’s why we are seeing patients, both patients that are willing to undergo the procedure earlier and also clinicians who are more comfortable offering it a little bit earlier in the onset of these kinds of complications, as opposed to waiting to the end or not the end, but just later on as the disease is really evolving and creating. And there are some things to be said about that. I mean, I think if patients are already experiencing significant balance issues or these kinds of really difficult to manage gait issues, the chance that we’re going to be able to help those is less compared to somebody who is maybe a little bit earlier in their disease course and hasn’t yet developed that degree of difficulty. So you know, it’s all a risk benefit analysis and very individual. And that’s, again, the whole reason that we go through these preoperative evaluations.
Soania Mathur:
No, and it makes so much sense. It applies to much of everything when it comes to treating Parkinson’s, it’s really individualized, has to go with the patient because we differ in so many ways. We’re similar in many ways, but different in many, many ways. So personalized medicine is important. What are some of the exciting advancements happening in DBS right now?
Joohi Jimenez-Shahed:
So again, I think some of the things that we’re seeing on the technology side are super exciting. And so we have now devices, like we said, with the remote programming capability, that’s a huge boon I think. We have devices that can also be used in a way that we can actually see the images of the patient’s brain with the electrode in place on this programming tablet. This is technology that is currently available in Europe should be coming to the US fairly soon. But that can also help us as clinicians in really kind of selecting those programming parameters more efficiently and maybe in a more individualized way as you were saying. We also have devices now that we briefly mentioned that can also sense brain signals. And what we are finding out on that front is that there are some very particular brain signals in Parkinson’s disease that are highly relevant to understanding how well we can control symptoms.
And so there’s a brainwave, if you want to put it that way, it falls into the category of what we call a beta band. And that’s a certain frequency of brain waves that we can measure. And which seems to correlate with symptoms of Parkinson’s. So the better your symptoms are controlled, then the lower this beta band activity is, or the less controlled your symptoms are the higher this beta band activity is. And we can actually measure that now with the deep brain stimulation devices. And it’s really interesting to see how we can now use that as a way to think about how to optimize the way that we program the devices. And so there’s opening up of like just a whole new kind of world of evolving DBS therapy that is based on understanding these brain signals and reacting to them, as opposed to just having a stimulator that’s on and kind of pumping, pumping, pumping.
We can actually modulate the way that that stimulation is delivered, or that’s the hope that we can do that in a way that becomes, you know, more efficient for patients, that better controls their symptoms, potentially has fewer side effects. So all of these different capabilities are really just allowing us to you know, make better decisions clinically for our patients. And I think in the end, it’s so much different now than it used to be even five years ago, the way that, you know, I sit in front of a patient with my programming tablet now. We have so much more information to use that can help us guide that programming. And I think in the end, you know, patients are going to get better results as a consequence of that.
Soania Mathur:
Yeah. It’s rapidly evolving that’s for sure. One thing I was wondering about, you know, there are always new evolving therapies. Well hopefully more in the future, but evolving therapies that come into play and some may be other types of brain surgeries or other treatments like stem cell placement and things like that, is DBS rather than lesioning that we used to do before, is it reversible? So like, would you say a new treatment comes out that you want to get, because you have DBS, would that necessarily preclude you from getting it?
Joohi Jimenez-Shahed:
Yeah, the answer is not necessarily, and it’s for the reasons that you’re kind of talking about. So DBS doesn’t necessarily just mean the act of putting the DBS in there doesn’t necessarily damage the brain per se. And so, you still have you know, kind of the other sort of normal brain anatomy, and we would expect people with DBS to sort of respond probably in the same way to some of these new and interesting therapies that are being considered, but you’re absolutely right. DBS can either be turned off or it can be taken out. There is small risk associated with taking it out, but it can be. And so if there is, you know, some new treatment, whether it’s stem cells or whether it’s gene therapy or whether it’s, you know, other kinds of immune treatments that are all currently under investigation you know, if we find out that these are really kind of disease altering and could be also beneficial for patients who already have deep brain stimulation devices in place, you know, we can certainly turn off the devices or take them out in order for patients to be able to access them.
Soania Mathur:
That’s good to know. I’m going to go to the chat to just take a couple of questions that people have posted. One is going back to the battery. As time goes on, the battery level required to maintain the same level of performance for the device goes up, is that correct, they’re wondering? And does that mean that each time when you replace, you have to go up and higher capacity?
Joohi Jimenez-Shahed:
So I think what that question might be getting at is yeah, over time we do tend to see the amount of stimulation, you know, like if you’re talking about an amplitude of stimulation, for example, that does tend to go up over time. I’m not sure if the question pertains to the need for battery replacements, but again, if you are increasing the amount of electricity that’s required to control the symptoms, that will have an implication for the battery life. There are also limitations to the stimulation in that regard. You can’t just ramp it up to 10 or 15, because most brains aren’t going to be able to handle that. So you have to be judicious in the amount of stimulation that you do apply. But generally speaking you know, the fact that we can adjust it in that and many other ways is actually one of the big advantages of stimulation over, as you said, those other surgical treatments.
Soania Mathur:
Great. Thank you. Someone was wondering, does DBS help any other Parkinsonisms like Parkinson’s plus or only idiopathic Parkinson’s disease, and someone else was wondering about it for focal dystonia say in your foot, that sort of thing.
Joohi Jimenez-Shahed:
Yeah. So I think with regards to the first question for atypical Parkinsonisms or Parkinsonisms that aren’t really Parkinson’s disease, DBS really does not have a role for those conditions. The brain changes in those patients really makes it so that even the stimulation can’t really help. So that’s part of the reason that we do these careful preoperative evaluations and why we focus so much on that levodopa response because we want to be sure that the symptoms that a person has responds to levodopa because it is regular garden variety Parkinson’s disease, because really those are the patients who are going to benefit and other people with atypical Parkinsonisms generally do not. As far as dystonia, for example, in a patient with Parkinson’s disease, usually dystonia is a symptom that occurs when people are OFF, right? So medications wear off, or maybe early in the morning before medicines kick in, those are common situations where patients may have dystonia. And again, because we are addressing the OFF symptoms with deep brain stimulation that actually does get better. And so that’s something to look forward to for individuals who are experiencing that. Cause I know that can often be quite uncomfortable and painful and affects walking. And generally if it gets better with levodopa, it will get better with the surgery as well.
Soania Mathur:
Yeah, it is quite uncomfortable. That’s for sure. A couple of questions about the actual procedure, how long does the actual surgery take and what about recovery time?
Joohi Jimenez-Shahed:
Yeah, so usually the surgery is done, again, it may vary from site to site, but generally there’s two procedures that have to be done. One is to get the brain electrodes in and the other one is to put the battery in and usually those procedures are separated. Meaning the brain electrodes go in first and then patients go home for a period of time and then come back and get the battery done. Some centers will do both sides of the brain in terms of placing the electrodes at once, some centers might do one side and then do the other side. So there is some variability there, but the first step is always putting those brain electrodes in. Usually that is something you have to get to the hospital early in the morning. You have to meet with the anesthesiologist, you have to get a pre-operative scan.
There’s a certain amount of waiting and planning that gets done. And then eventually you get into the operating room, the time in the OR will depend on whether one side or two sides are being done. If it’s one side, it could be, you know, three or four hours in the operating room, it could be longer if both sides are being done. And again, may vary according to the surgeon and how smooth the operating room runs. But it’s usually then followed by a stay in the post anesthesia care unit. And the patient will then usually be transferred to a regular part of the hospital overnight for observation and provided everything goes okay overnight and there’s a follow-up scan that doesn’t show any complications from the procedure, patients go home the next day after the brain surgery. So it’s a one-night hospital stay, does not require going to rehab, doesn’t require any sort of very extensive restrictions on functioning and activities and things like that. And then when the patient comes back for the battery placement, that’s usually again a day surgery. So you go in in the morning, you get the battery placed, they kind of uncoil everything that they’ve put under the scalp. They tunnel it under your skin and connect it to the battery. So that one is actually done under general anesthesia, but it’s a much shorter procedure. And so patients can recover from that in the anesthesia and go home the same day after that one is done. So not a whole lot of downtime.
Soania Mathur:
What is the success rate someone else had wondered?
Joohi Jimenez-Shahed:
Yeah, very complicated question. I think when we are doing our job on our side, meaning thinking of the right patients and selecting the right patient, doing all those preoperative assessments and things like that. And then you have a surgeon who gets it into the right place and you have a programmer who knows how to program it, then the results can be quite good. And what we would generally expect to see are significant reductions in those fluctuations. So the ON time should improve and become more consistent, the dyskinesias should go away, the tremor should get better controlled and whatever your best state on levodopa prior to surgery was, should be what you’re experiencing most of the day after DBS surgery minus the dyskinesias. So I think, you know, when all of those steps kind of go well, then that’s the result that we look for. And thankfully you know, I think at the vast majority of centers, this is the type of result that patients will get. Again, as we talked about earlier, very important to kind of do a little bit of research and ask your questions and feel comfortable with how your team approaches the care of patients with DBS. But I think that is definitely an outcome that we see fairly regularly when we’re applying all those criteria.
Soania Mathur:
That’s great. This is actually an interesting question. We’ve talked a lot about the advancements that are being made. So if you get a certain DBS system, can you upgrade?
Joohi Jimenez-Shahed:
Yeah, so there are three components to the hardware. There’s a brain wire, which is actually, you know, the part that’s in the brain in the deep brain structures that control movement. There’s an extension wire and there’s a battery. So I think changing brain electrodes is a big ask. That requires a repeat brain surgery. And so probably not something that we would recommend unless there was some reason that that system needed to be explanted to begin with. So maybe it wasn’t in the best location. Maybe there was infection, things that don’t happen very commonly. Those are situations where we’ve certainly had patients switch from one device to another, but the brain electrode, unfortunately, well not, fortunately or unfortunately it’s just not something you want to go in and switch out for without a really good reason to do that. The batteries are a slightly different issue. Some of the system components are able to be used in combination across manufacturers, but many of them are not. And so it may not be the case that you could just get a battery switch and take advantage of some of the different capabilities out there just because the devices themselves are not interchangeable in that way with some few exceptions.
Soania Mathur:
There’s also a few questions about lifestyle changes that you have to have or have in place if you get DBS surgery, could you just maybe review that?
Joohi Jimenez-Shahed:
I assume maybe the question is pertaining to the kind of postoperative period?
Soania Mathur:
I think more kind of the physical activities that you might not be able to do once you have DBS going through airports security, that sort of thing.
Joohi Jimenez-Shahed:
Yeah. So I think activity-wise part of the reason we’re doing deep brain stimulation is because we want you to be able to maintain or even improve your level of activity. So hopefully there’s more things that you can do after DBS compared to before at least that would be our hope. I think, you know, be smart about what you do. Certain things are, you know, there’s warnings and sort of cautions and precautions that are listed for things like scuba diving or I think there’s even kind of a warning about swimming after deep brain stimulation and not necessarily because there’s an across-the-board risk of swimming. There were just some case reports, I think, where individuals felt like they had less ability to swim safely. And there were some, I think accidents associated with that.
And so there’s no warning associated with that. So we recommend that people are with somebody if they want to go swimming so that they’re not caught in that kind of situation unawares. There, I think welding, you shouldn’t weld after having a deep brain stimulation, there’s certain types of welding that are restricted, but of course those are things that hopefully we would know going in ahead of time. Aside from that. I mean, not really. I’ve had patients do a remarkable number of things after DBS, whether it’s athletics, whether it is you know, recreational activities. And so I think again, just be smart, you’ve got a pretty pricey piece of equipment in there, so you’d want to be mindful of that and certainly protective of it. You don’t want to do anything that involves a lot of I don’t know, bumping or hitting or things like that. So I think some practical things are probably an order, practical considerations. Sure. Not a whole lot of direct contraindications that are common activities actually.
Soania Mathur:
Ok, good to know. And one sort of, maybe one of the last questions for our chat, is the decision of specialists and type of device up to the insurance providers? And is it covered by Medicare? So questions about cost of this whole thing.
Joohi Jimenez-Shahed:
So this is a standard of care surgery. So it is covered by insurance, including Medicare, you know, precise insurance coverage may be determined by your specific plan or out of pocket. You know, things like that. You’ll have to talk specifically to the surgeon’s office about that, and they can give you those estimates before you go in, but it is a covered surgery. And most insurances are going to require the information about those preoperative assessments. They’re going to want to see the OFF and the ON, they’re going to want to see the neuropsychology testing. And so that is part of their sort of approval. So they know that people are being carefully considered and, you know, really are likely to benefit from the procedure. But I think that’s kind of fairly standard practice at most places that do the surgery. And so if all of those things are done and documented fine, then there really should not be any issue getting it covered.
Soania Mathur:
That’s good to know as well. So as our time comes to an end, I’d like to ask you maybe one last question. What is the most exciting potential advancement coming down the pipelines? Like what makes you most excited that may be coming to help patients in the not too distant future?
Joohi Jimenez-Shahed:
I think we kind of touched a little bit on some of these advancements and I think it’s really kind of a tremendous time for DBS. I think we’re finding all these different ways that we can really hone in on the way that the stimulation should be programmed and how it can help. And I think you know, we’re studying more about the brain circuits that are being affected by deep brain stimulation. That’s one of the things that we do at our center, trying to understand how can we treat more than just motor symptoms of Parkinson’s. So are there really more direct ways to address some of the other problems that come along with Parkinson’s disease using that same stimulator without having to do too much differently in terms of you know, the surgery itself or implanting the device. So I think, you know, it’s allowing us to understand more about Parkinson’s disease.
It’s allowing us to understand more about how we can get good control of symptoms. And I think all of this is really kind of capable because of these advances in imaging and in programming and in sensing all of the different things from all the different manufacturers actually each in their own way, are helping us to approach this disease in a much better way. And I think we’re going to see some very different approaches to how we can program patients and how they can benefit from these stimulators in a way that is, you know, more comfortable perhaps or more consistent and maybe, you know, by limiting some of these side effect issues, or maybe even limiting the amount of time between battery replacements, for example. So a lot of efficiencies, a lot of improvements, a lot of better understanding of disease. And I think that’s you know, really kind of an amazing place to be right now
Soania Mathur:
And all of this sort of working towards bettering a patient’s quality of life, which is so important, so important. So thank you also kindly for joining us today. I hope you found the discussion helpful educational. I know I certainly did. And thank you, Dr. Shahed for your time and expertise. It was a great discussion and really enjoyed speaking with you. And remember everyone, we may not have a choice in our diagnosis, but how we face the challenges this disease brings is really ours to define. So choose to optimize your quality of life, educate yourself, empower yourself and celebrate your daily victories.
Show Notes
What is DBS and how does it work? DBS stands for deep brain stimulation, a surgical therapy during which a battery and wires are placed in the brain, with electrodes placed on areas in the brain that are responsible for movement. After the surgery, the device is activated and programmed over a period of four to six months. The device sends electrical impulses to these motor areas to correct for this circuitry not firing correctly on its own in Parkinson’s.
How does someone know they’re ready for DBS? It depends on the individual, but DBS is often recommended for people who meet certain requirements when medications are no longer managing OFF times effectively or there is a high occurrence of tremor, dyskinesia, or dystonia.
Who is the ideal candidate for DBS? In general, the criteria for receiving DBS are the following:
-
- The person’s symptoms should be responsive to levodopa. This is typically the case in idiopathic Parkinson’s, but is not always the case in Parkinsonism, essential tremor, or Atypical Parkinson’s. If your symptoms do not respond to levodopa, they will not respond to deep brain stimulation.
- While experiencing non-motor symptoms such as depression, anxiety, or cognitive difficulties such as memory loss does not rule someone ineligible for DBS, medical professionals may proceed with extra caution in these cases, as there may be a risk of these symptoms worsening after the surgery.
- If someone has many other medical conditions, DBS may or may not be an option, as co-morbid conditions may affect the effectiveness of the device or the safety of the procedure.
Is there an age limit for getting DBS? Although there have been suggestions in the past that people over 70 should not get DBS, medical professionals now primarily look at the above criteria to determine if DBS is a good fit.
Are there any non-motor symptoms that improve with DBS? Although non-motor symptoms may not be directly affected by DBS, the therapy does reduce OFF times, and this may result in fewer non-motor symptoms.
What is the history of DBS? The practice of deep brain stimulation has evolved over time, but all started with an accidental discovery that found that people with Parkinson’s who had also had a stroke in a particular region of the brain had a reduction in their Parkinson’s motor symptoms after undergoing brain stimulation.
What types of DBS devices are available? All DBS devices have the same basic components, but there are slight variations in the “bells and whistles” based on the manufacturer. These differences include extra precise control of electrical impulses, the ability to steer the electrical current in a particular direction, and the ability to sense and record electrical signals. DBS devices may also offer differences in battery type, with some featuring replaceable batteries and others rechargeable batteries.
Who decides which device you get? It may vary from place to place. People undergoing DBS are welcome to voice their preferences, but there may be some limitations as to which device you get based on the facility, the surgeon performing the surgery, and your insurance.
How does someone go about finding and selecting a neurosurgeon to do the surgery? Usually a referral to a surgeon will come from your neurologist, but as a person with Parkinson’s, it is important to feel empowered to ask additional questions to establish confidence in your surgeon. Some questions you may ask are:
-
- How many DBS cases has the surgeon done?
- How familiar is the surgeon with the procedure?
- What is the surgeon’s complication rate and/or rate of side effects?
What are the pros and cons of different types of batteries? The conventional battery life depends on the programming that’s required to control the symptoms. The average life of a conventional battery is three to five years. Rechargeable batteries are currently estimated to last up to 25 years, but rechargeable batteries require regular re-charging approximately once a week.
How long do the non-rechargeable batteries last and what does the process look like to replace the battery? Non-rechargeable batteries tend to need replacing every three to five years. When replacing a battery, you will likely go under general anesthesia, and the surgeon will go in through the same incision used to originally place the battery. The procedure typically lasts about an hour, and most people having the surgery will go home the same day.
What does programming a DBS device look like? When you get DBS, it is not as simple as turning it on. Following the surgery, it typically takes four or five sessions over the course of approximately six months to program your DBS with a specialist.
What are DBS options for individuals who live in more remote areas? Remote programming now exists for certain DBS devices, which allows the user to program their device at home during a virtual visit with their physician. This process does, however, require a good internet connection, which may be a challenge for some people living in rural communities.
What are some of the limitations of the technology? The most important thing to recognize is that DBS is not a replacement for your medications. DBS works in conjunction with your medications to reduce OFF times, reduce dyskinesia, and increase ON times. Parkinson’s will continue to progress, and though increasing stimulations may help to control the progression of these symptoms, DBS does have its limitations.
Is there an advantage to getting DBS earlier in the course of Parkinson’s? In the past, it was suggested that people should only receive DBS as a last resort. This is no longer the case, and in general, the guidelines for receiving DBS are based on a general set of criteria rather than length of time since diagnosis or age.
What are some of the exciting advancements happening in DBS right now? This is an exciting time in the advancement of DBS. Some of the most recent advancement include devices that have remote programming capability, new brain imaging devices, and new devices that can sense and track brain signals, which may be helpful in eventually understanding the brain activity for certain symptoms, and therefore, optimizing treatment.
Is DBS reversible? Yes, DBS devices can be turned off or taken out.
How long does the surgery take? The surgery is usually split into two, sometimes three, procedures. The first procedure involves placing the brain electrodes (sometimes the surgeon will split this into two surgeries, one for each side of the brain). After this procedure, most people will be required to stay overnight to ensure there are no complications. Then, there will be a follow-up procedure to place the battery. This surgery typically involves a general anesthetic, and the people undergoing the procedure is typically in and out of the hospital the same day.
What is the success rate of DBS? Generally, success rates are very high. The expected result is to see the same reduction of symptoms that you once had when your medications were working optimally.
Can you upgrade your DBS system? Swapping out electrodes would likely not be recommended due to the invasiveness of the surgery. If there were to be new advancements in batteries, you may or may not be able to swap this out, based on its compatibility with your primary device.
What is the cost of DBS and is it covered by insurance or Medicare? DBS is considered a standard of care surgery and so is typically a covered surgery, Medicare included. Specific costs will be based on your insurance coverage.
Additional Resources
The What, Why, How, and More of DBS for the Newly Diagnosed
[Webinar Recording] A DBS Forum
Life Before and After Deep Brain Stimulation
[Webinar Recording] Your DBS Questions Answered
When DBS Powers Down: A Personal Account
more about the speaker
Dr. Joohi Jimenez-Shahed is the Medical Director of Movement Disorders Neuromodulation & Brain Circuit Therapeutics at the Icahn School of Medicine at Mount Sinai. After completing her undergraduate degree at Washington University in St. Louis, Dr. Jimenez-Shahed received her medical degree from Baylor College of Medicine (BCM) and Neurology residency training at Duke University Medical Center. She then completed a fellowship in Movement Disorders at the Parkinson’s Disease Center and Movement Disorders Center at BCM. Her research interests lie in investigating the intraoperative neurophysiology of patients undergoing deep brain stimulation (DBS) for movement disorders and the application of wearables and digital health technologies to the care of patients with Parkinson’s disease.
THANK YOU TO OUR GOLD-LEVEL SPONSORS*
Live Well Today Webinar Series Presenting Partners*
 *While the generous support of our sponsors makes our educational programs available, their donations do not influence Davis Phinney Foundation content, perspective, or speaker selection.
*While the generous support of our sponsors makes our educational programs available, their donations do not influence Davis Phinney Foundation content, perspective, or speaker selection.